
:max_bytes(150000):strip_icc()/the-periodic-table-of-the-elements-in-a-school-in-san-francisco--california--75471038-5966b47e5f9b58161833170a.jpg)
The following groups are included in the p block of periodic table. Now, here is your p block on the Periodic table. Here, you will exactly come to know how Henry Moseley arranged the elements in periodic table on the basis of modern periodic law.
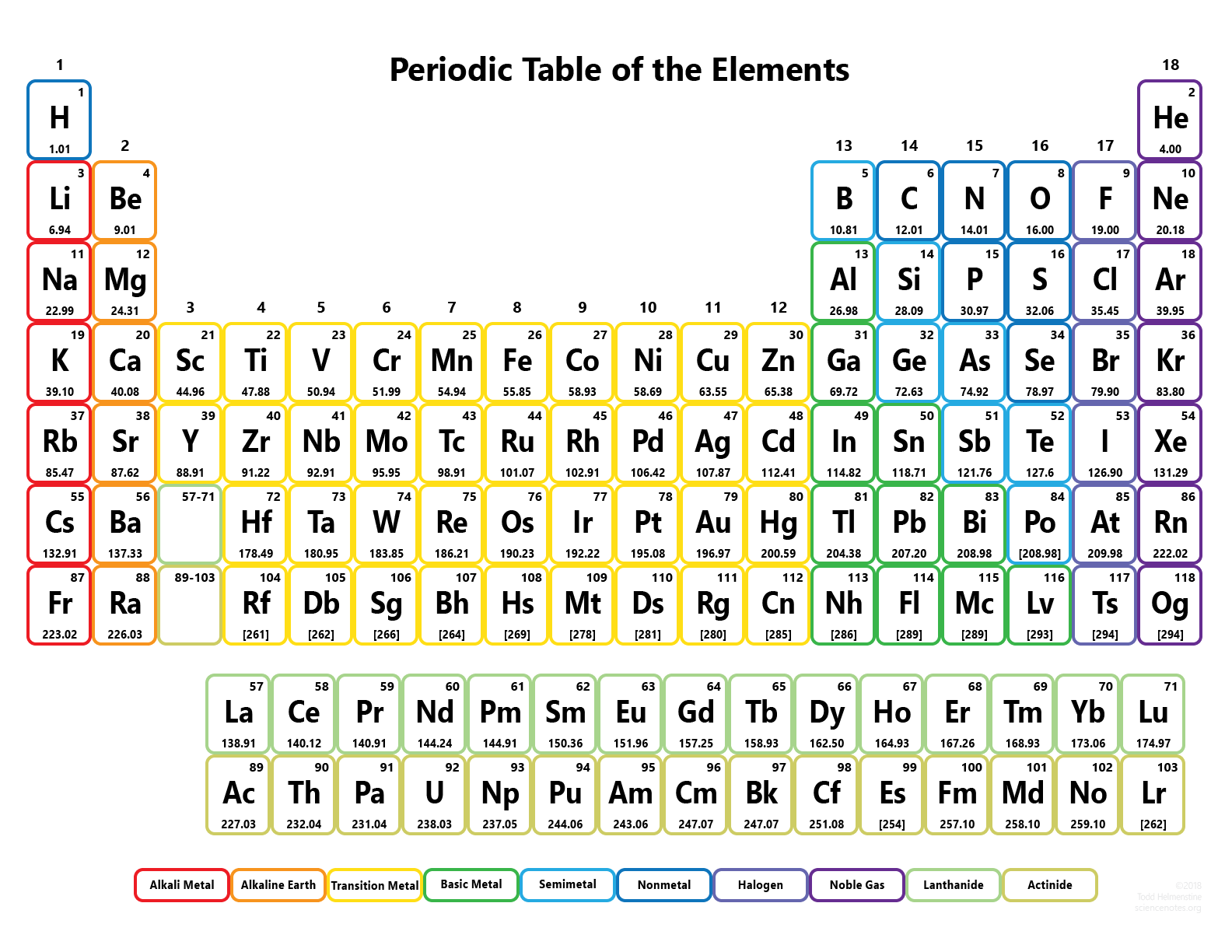
How are elements arranged in periodic table? Modern periodic law says that the properties of elements are the Periodic function of their atomic number.Īccording to the modern periodic law given by Henry Moseley, the elements should be arranged in the table on the basis of their ATOMIC NUMBER.Īfter arranging all the elements based on atomic number, the modern periodic table came into existence.Mandeleev law says that the properties of elements are the functions of their atomic mass.Henry Moseley arranged the elements according to the increasing atomic number.Mendeleev arranged the elements according to the increasing atomic mass.This law is exactly similar to the Mendeleev law, but the only difference is Modern Periodic Law was given by Henry Moseley in 1913. The properties of the elements are the Periodic function of their ATOMIC NUMBERS. Yes, all the elements in modern periodic table are arranged on the basis of their ATOMIC NUMBER. We have seen in previous section that Dobereiner’s law, Newland’s law as well as Mendeleev law were based on the arrangements of elements according to their increasing atomic masses.īut the only difference in modern periodic law is I have also discussed the merits and demerits of the Mendeleev periodic table. I know this seems a little difficult for you, so you can refer to this detailed guide on “ Mendeleev periodic table” where I have explained all these things with proper images for your better understanding. So there were some empty blocks seen on the Mendeleev periodic table. Now look, while arranging the elements according to atomic masses, Mendeleev found that the properties of few elements were not matching with any of the previous ones, so he didn’t place such elements in that particular column. Why are some blocks empty in the above Mendeleev periodic table? That’s why Mendeleev placed those elements in the same block. The short answer: Those elements show similarly in their properties. Have you noticed that many blocks have more than one element in Mendeleev periodic table? Why? There are a total 8 vertical columns in the Mendeleev periodic table, which are known as groups.Īnd there are 7 horizontal rows, which are known as periods.

This table is known as the Mendeleev periodic table of elements.


 0 kommentar(er)
0 kommentar(er)
